INDWELLING/FOLEY CATHETERS & ACCESSORIES
From Tried-and-True to
Truly Innovative
A complete range of Foley essentials delivering convenience and outstanding value.
HCPCS: A4338, A4340, A4344, A4346
See product description for specific code.
More Products, More Options for Optimal Outcomes
The HR HealthCare indwelling product portfolio is designed to support healthcare professionals with everything needed for indwelling catheterization procedures, balancing efficiency, quality, and cost—without compromise
Insertion Trays
TruAdvance® and TruCath® Duo Foley Insertion Trays Incorporate all the prep and procedural components for insertion.
Five configurations available.

Securement
TruLock™ Foley Catheter Securement Devices are designed to provide reliable Foley catheter stabilization and decrease the likelihood of dislodgement.
Each device includes a CliniCare™ Skin Protectant Wipe for skin protection and optimal adhesion.
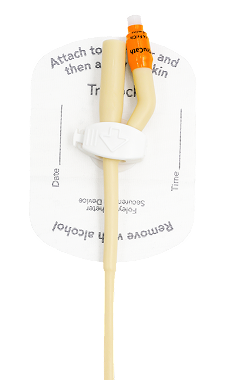
Clamp
Universal 2-way configuration. Swivels 360° for patient comfort and safety.
Base Material
Mesh-cloth with medical-grade adhesive—provides secure wear
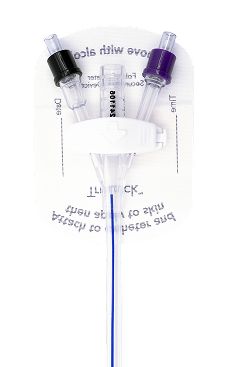
Clamp
Universal 3-way configuration. Swivels 360° for patient comfort and safety.
Base Material
Mesh-cloth with medical-grade adhesive—provides secure wear
Drainage
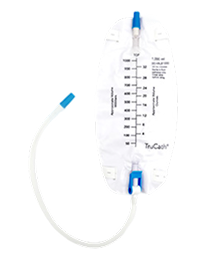
TruCath® Leg and Night Drainage Bags, available in multiple volume capacities, provide dependable and convenient urine collection.
Flocked back with preattached straps
Eyes
Large and oval-shaped to support maximum drainage
Leg Bag
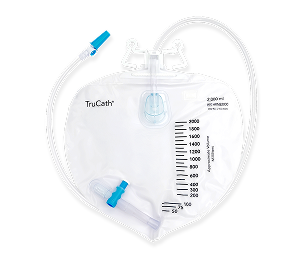
Double hanger secures the bag to a walker, wheelchair, or bed
Night Bag
Indwelling Catheters
The TruCath® Foley range includes integral and innovative configurations to accommodate care. Connect to a TruCath® leg or drainage bag for urine collection.

Balloon
Pretested and inspected for integrity and position
Eyes
Large and oval-shaped to support maximum drainage
Latex Core
Provides flexibility for patient comfort
Silicone-Elastomer Coating
Helps reduce the potential for encrustation and mucosal irritation
Securement
Secure the catheter with a TruLock™ Foley Catheter Securement Device
Drainage
Connect to a TruCath® Leg Bag (500 or 1000 ml) or TruCath® Night Drainage Bag (2000 or 4000 ml)
Inflation
Inflate with an AquaFlate® Sterile Water Syringe included in all TruAdvance® Foley Insertion Trays
Balloon
Pretested and inspected for integrity and position
Inner Lumen
Promotes a higher flow rate compared to latex
Silicone
Material provides chemical and thermal stability, low surface tension, and hydrophobicity
Securement
Secure the catheter with a TruLock™ Foley Catheter Securement Device
Drainage
Connect to a TruCath® Leg Bag (500 or 1000 ml) or TruCath® Night Drainage Bag (2000 or 4000 ml)
Inflation
Inflate with an AquaFlate® Sterile Water Syringe included in all TruAdvance® Foley Insertion Trays

Bladder-Protection Balloon
Helps provide a cushion for the collapsed bladder
Bladder-Retention Balloon
Provides secure placement in the bladder
Inner Lumen
Promotes a higher flow rate compared to latex
Silicone
Material provides chemical and thermal stability, low surface tension, and hydrophobicity
Securement
Secure the catheter with a TruLock™ Foley Catheter Securement Device
Drainage
Connect to a TruCath® Leg Bag (500 or 1000 ml) or TruCath® Night Drainage Bag (2000 or 4000 ml)
Inflation
Inflate with an AquaFlate® Sterile Water Syringe included in the TruCath® Duo Insertion Tray
Inflation
Inflate with an AquaFlate® Sterile Water Syringe included in the TruCath® Duo Insertion Tray
Variety for Care, Innovation to Enhance
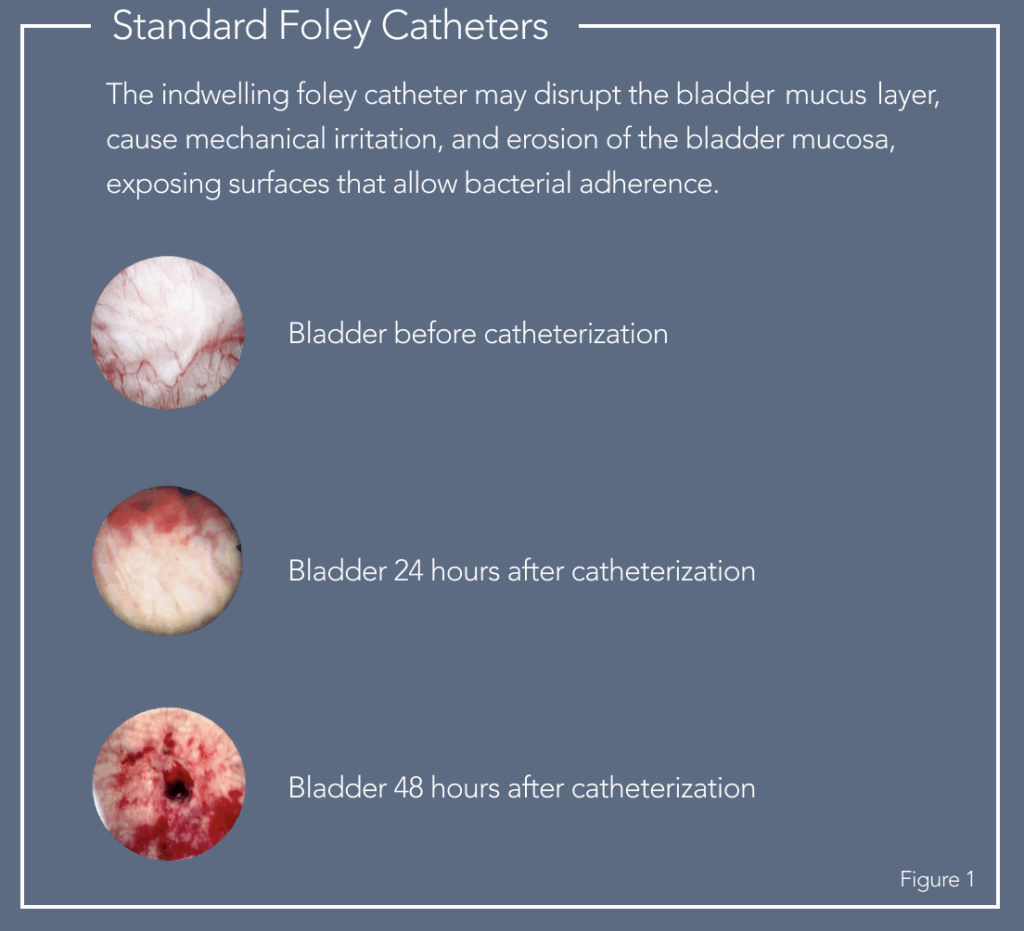
Patients face potential non-infectious and infectious complications with indwelling catheterization, particularly when used long-term, including pain and discomfort, tissue damage, hemorrhage, encrustation of the catheter leading to blockage, and lower urinary tract infection.
The innovative design of the TruCath® Duo Dual-Balloon Urinary Catheter aims to minimize trauma to the bladder mucosa, thereby helping to reduce the risk of bladder spasms, often caused by irritation, inflammation, or constant stimulation from the catheter. Additionally, by lowering mucosal irritation and providing a surface less conducive to bacterial adhesion, TruCath Duo may help reduce the risk of catheter associated urinary tract infections (CAUTIs), a common complication of indwelling catheter use.
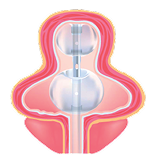
Distal balloon acts as a cushion for the collapsed bladder
References: 1. Norden CW, Green GM, Kass EH. Antibacterial mechanisms of the urinary bladder. J Clin Invest. 1968 Dec;47(12):2689-700. doi: 10.1172/JCI105952. PMID: 4881768; PMCID: PMC297440.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC297440/ 2. Parsons CL, Mulholland SG. Bladder surface mucin. Its antibacterial effect against various bacterial species. Am J Pathol. 1978 Nov;93(2):423-32. PMID:
362941; PMCID: PMC2018387. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2018387/ 3. Parsons CL, Mulholland SG, Anwar H. 1979. Antibacterial activity of bladder surface mucin duplicated by exogenous
glycosaminoglycan (heparin). Infect Immun 24:.https://doi.org/10.1128/iai.24.2.552-557.197 4. COX CE, HINMAN F Jr. Experiments with induced bacteriuria, vesical emptying and bacterial growth on the
mechanism of bladder defense to infection. J Urol. 1961 Dec;86:739-48. doi: 10.1016/S0022-5347(17)65257-1. PMID: 13881887. https://pubmed.ncbi.nlm.nih.gov/13881887/ 5. Wilson, Mary. (2008). Causes and
management of indwelling urinary catheter- related pain. British journal of nursing (Mark Allen Publishing). 17. 232-9. 10.12968/bjon.2008.17.4.28712. https://www.researchgate.net/publication/5438940_Causes_
and_management_of_indwelling_urinary_catheter-_related_pain 6. Saint S, Trautner BW, Fowler KE, et al. A Multicenter Study of Patient-Reported Infectious and Noninfectious Complications Associated With
Indwelling Urethral Catheters. JAMA Intern Med. 2018;178(8):1078–1085. doi:10.1001/jamainternmed.2018.2417. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2686144#google_vignette
Figure 1: Dr. Bruce E. Wiita, M.D., F.A.C.S., 2012, digital images.
HR HealthCare Products
TruCath Silicone-Elastomer Coated Latex
Indwelling catheters featuring a latex core for flexibility and patient comfort while the silicone-elastomer coating helps reduce the potential for encrustation and mucosal irritation.
TruCath 100% Silicone
Indwelling catheters featuring a 100% silicone material to support safety for patients with latex sensitivity or latex allergies.
TruCath Duo 100% Silicone Dual-Balloon
Designed to help address frequent challenges posed by standard Foley catheters, such as the risk of bladder mucosa damage, the occurrence of bladder spasms, and the potential for infection.
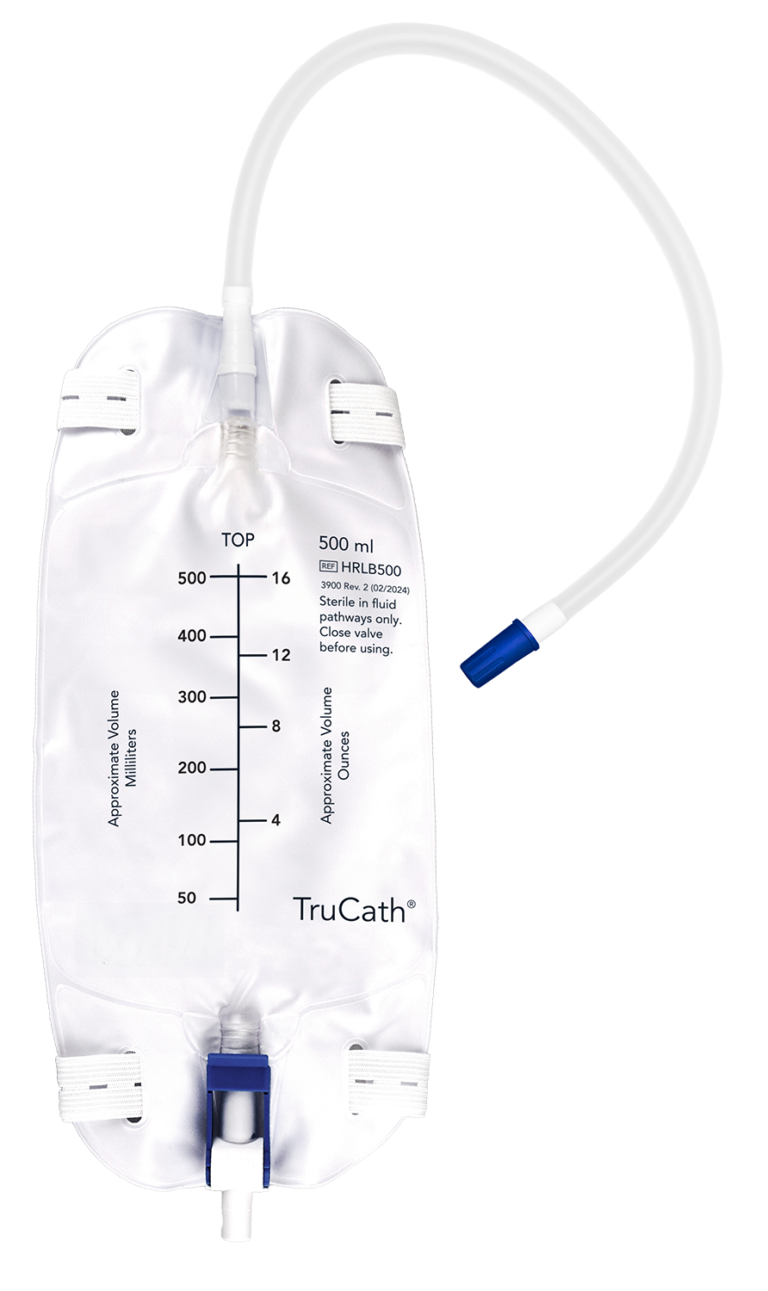
TruCath Leg Bags
Available in 500 ml and 1000 ml sizes,
this product features a flocked back, a convenient flip-drainage port, and comes with preattached leg straps.
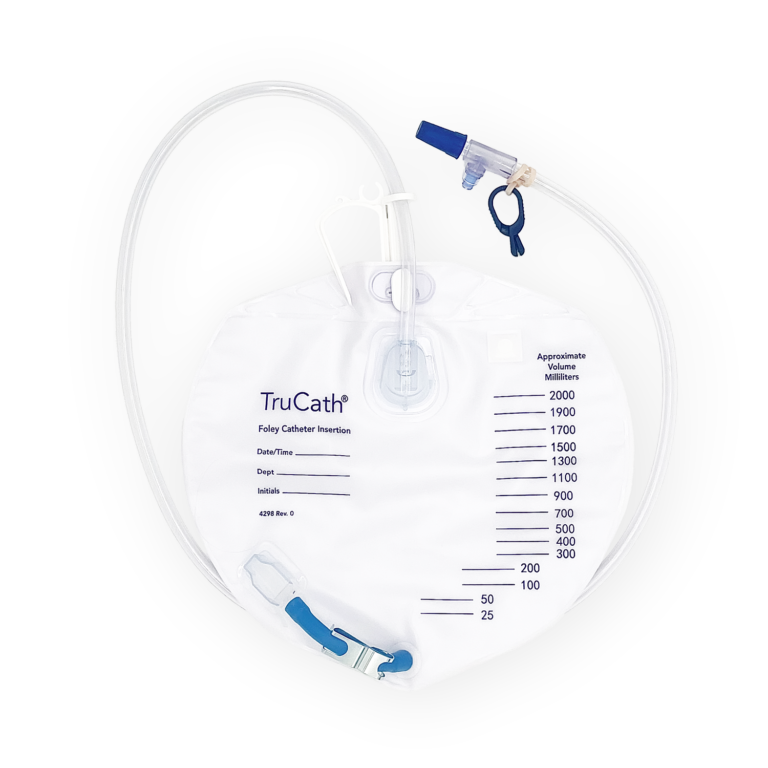
TruCath Night Drainage Bags
Available in 2000 ml and 4000 ml sizes, this diamond-shaped product features a T-tap drainage valve, a sample port, and a double hanger for secure attachment to a walker, wheelchair, or bed.
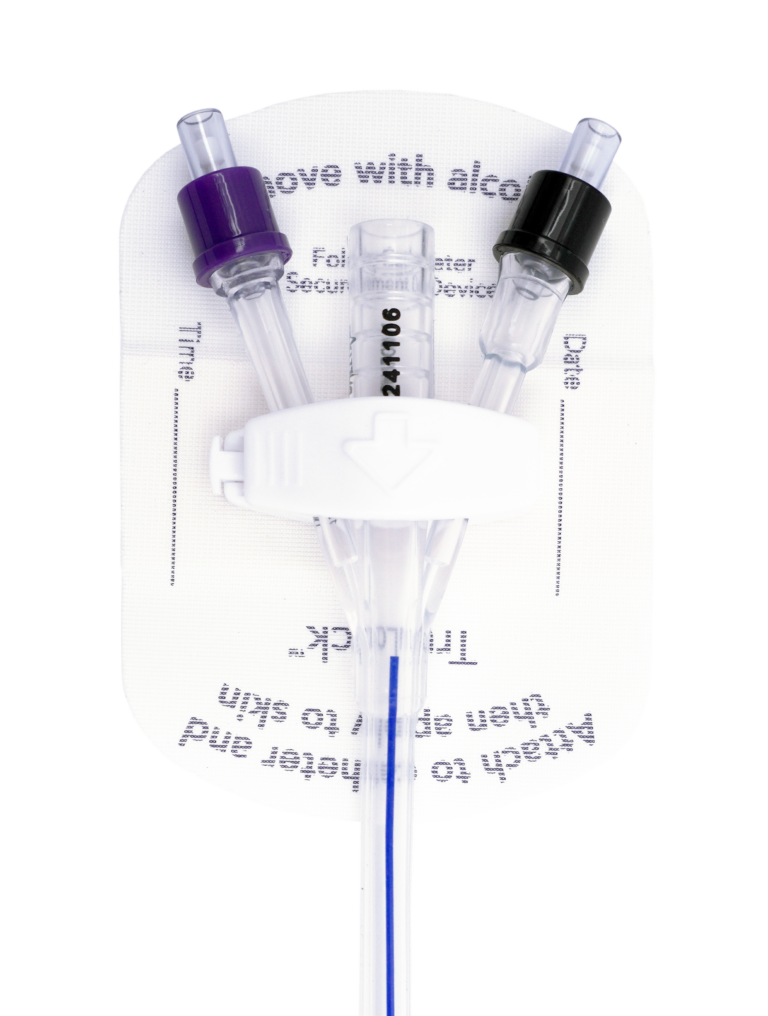
TruLock Foley Catheter Securement Device
Designed to hold a Foley catheter in place and support correct positioning in the bladder for urinary drainage.

TruAdvance | TruCath Duo Foley Insertion Trays
Provides all the necessary prepping components for aseptic insertion of an indwelling catheter.
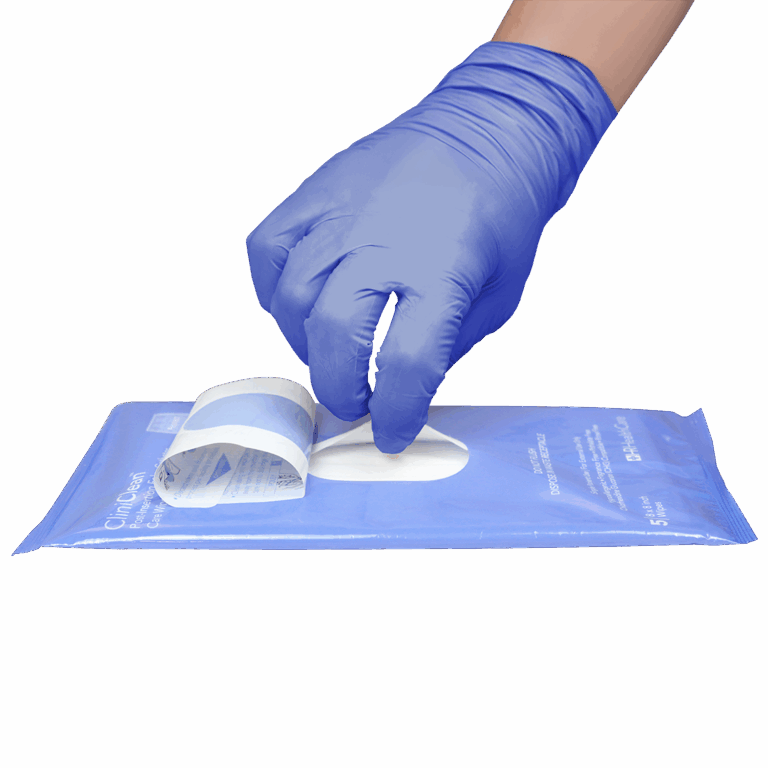
CliniClean Post-Insertion Foley Catheter Care Wipes
A five-wipe cleansing pack designed to simplify and standardize routine Foley catheter care.

